From the depths of the Earth to the skies above Hiroshima, uranium has played a dramatic role in human history. But how is this raw element transformed from rock into one of the most destructive forces ever engineered? In this article, we’ll walk through the entire process—from uranium extraction to nuclear chain reactions—using simplified explanations and illustrative concepts inspired by animations.
1. Extracting Uranium: From Ore to Chamber
The process begins with uranium ore, a dense rock containing trace amounts of uranium. Using precision drilling, engineers pull a reamer through a pilot hole, expanding the opening and allowing the ore to break free. The crushed rock falls into what’s known as an extraction chamber.
To avoid risk from collapsing rock, a remotely operated bulldozer collects the uranium-rich material. This is one of the first safety measures in a process that gets increasingly complex—and dangerous.

2. From Rock to Gas: Preparing for Enrichment
Once collected, the uranium ore is crushed and ground into a fine powder. It’s then chemically processed into a compound called uranium hexafluoride (UF₆). This compound becomes a gas when heated, making it ideal for centrifuge-based enrichment—a critical step for both nuclear power and weaponization.
3. Centrifuge Enrichment: Separating the Isotopes
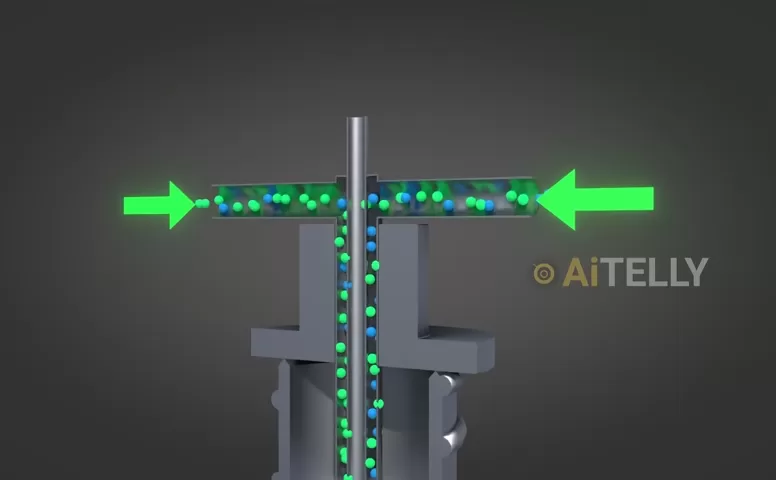
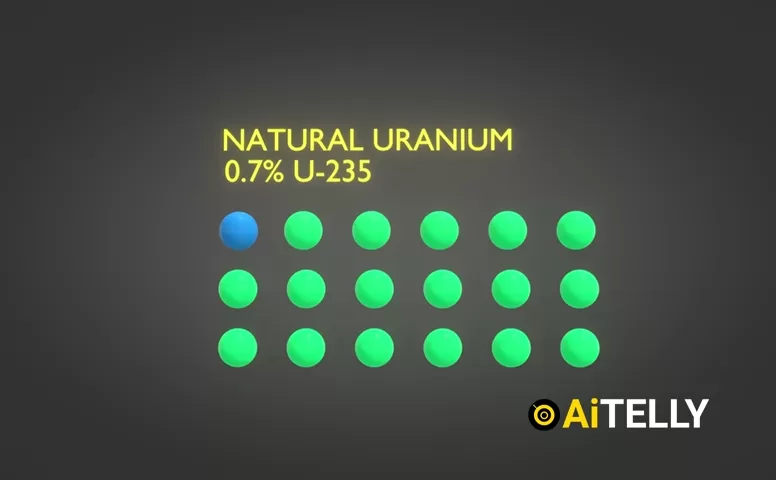
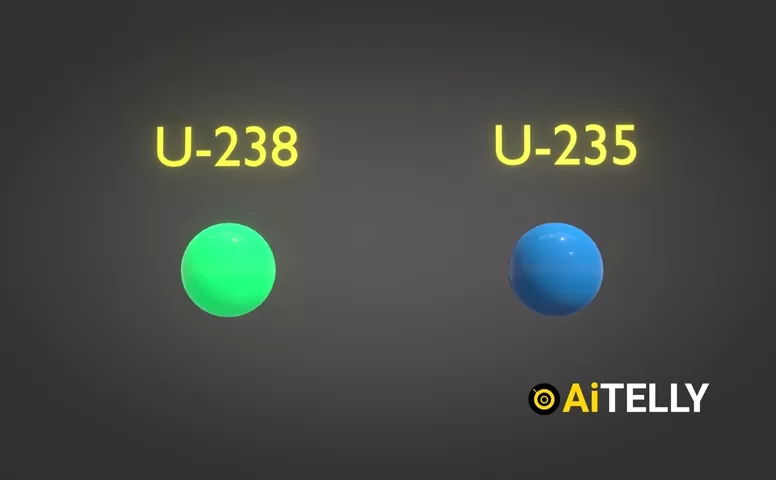
Natural uranium contains about 0.7% uranium-235 (U-235) and 99.3% uranium-238 (U-238). The challenge? Only U-235 is fissile—it can sustain a nuclear chain reaction. To concentrate it, scientists use a centrifuge.
How It Works:
- The uranium hexafluoride gas is fed into a high-speed spinning cylinder.
- Due to centrifugal force, the heavier U-238 atoms (green dots) migrate to the outer wall.
- The lighter U-235 atoms (blue dots) remain closer to the center.
- After repeated cycles, the U-235 concentration increases.
Enrichment Levels:
- Natural uranium: 0.7% U-235
- Low-enriched uranium (LEU): ~5% U-235 (used in nuclear reactors)
- Weapons-grade uranium: 90%+ U-235 (used in nuclear weapons)
4. Engineering a Nuclear Weapon: The “Little Boy” and “Fat Man”
Two major atomic bomb designs emerged during World War II: Little Boy and Fat Man.
Little Boy:
- Type: Gun-type uranium bomb
- Length: 10 ft (3 m)
- Diameter: 28 in (71 cm)
- Fissile material: U-235
Fat Man:
- Type: Implosion-type plutonium bomb
- Length: 10.8 ft (3.3 m)
- Diameter: 60 in (1.5 m)
- Fissile material: Plutonium-239
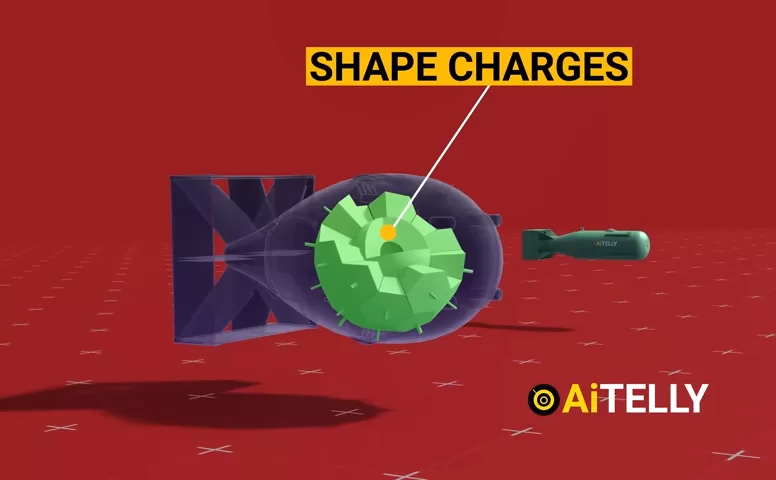
5. Deep Dive: Little Boy Gun Design
Let’s explore the inner workings of the Little Boy atomic bomb, which was dropped over Hiroshima in 1945.
Key Components:
- Electric primers: Initiate the propellant charge.
- Cordite bags: Four units containing 2 lbs (0.9 kg) each of explosive powder.
- Projectile rings: Hollow slugs of U-235 (~84 lbs).
- Target rings: Solid U-235 (~56 lbs).
- Gun barrel: 6.5 in (170 mm) smooth-bore barrel.
- Polonium initiators: Ensure a nuclear chain reaction upon impact.
Functionality:
- Once armed, the explosive charge propels the U-235 projectile into the target rings.
- The rapid joining of the two U-235 masses forms a supercritical assembly.
- Neutrons from the initiators trigger fission, creating a massive explosion.

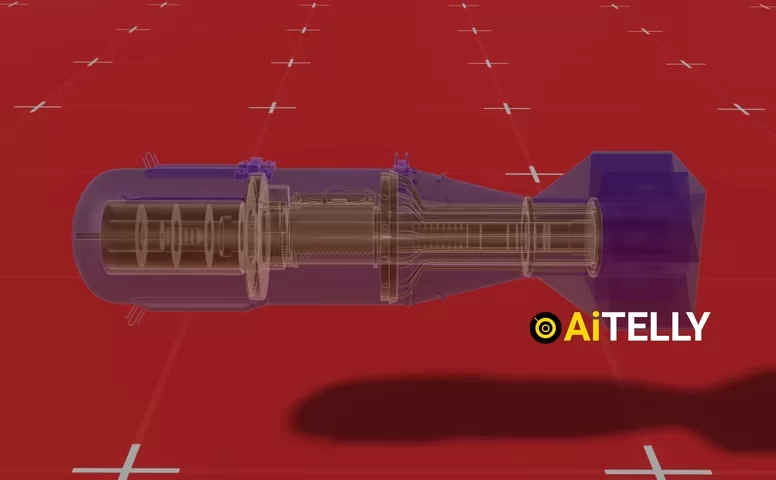
6. The Role of the Barometer and Radar Altimeter
The Little Boy bomb used both barometric sensors and radar altimeters to determine when to detonate.
- Barometric ports measured air pressure to approximate altitude.
- Radar altimeters bounced signals off the ground to get precise readings.
- When 1,900 ft (580 m) was reached, the internal circuitry initiated the firing sequence.
Why an airburst? Detonating above the ground maximized blast damage while minimizing cratering—crucial for strategic impact.

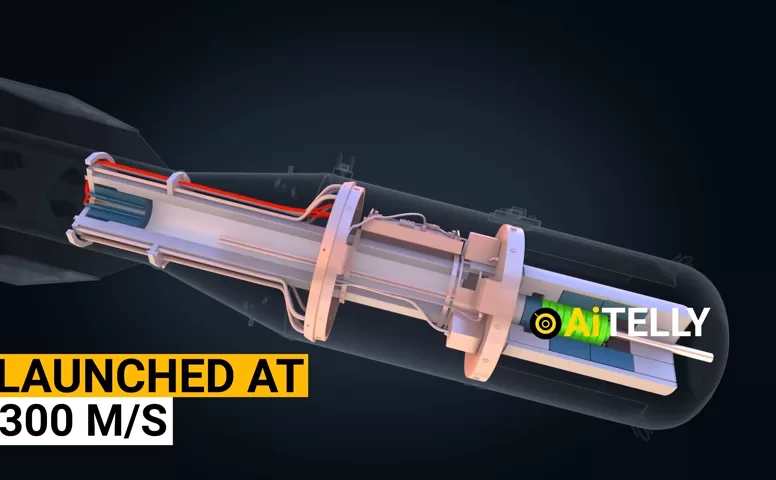
7. Step-by-Step Detonation Sequence of Little Boy
Here’s the simplified breakdown of what happens inside the bomb during deployment:
- Arming: Three safety plugs are removed manually by a crew member.
- Release: The bomb is dropped from the aircraft.
- Power switch: It switches to its internal 24V battery.
- Barometer triggers: At ~580 meters, circuits close.
- Radar altimeter confirms: Final altitude verification.
- Electric primers ignite: The gunpowder charge is set off.
- Projectile launched: U-235 slug travels down the barrel at ~300 m/s.
- Fission initiates: Polonium triggers the chain reaction.
- Explosion: A nuclear detonation occurs within milliseconds.
8. The Chain Reaction: How Fission Works
Here’s a highly simplified version of what happens on an atomic level:
- A neutron strikes the nucleus of U-235.
- The nucleus absorbs the neutron and becomes unstable.
- It splits into two smaller nuclei, releasing 2–3 more neutrons and huge amounts of energy.
- These new neutrons strike more U-235 atoms, continuing the chain reaction.
This self-sustaining reaction is what powers both nuclear reactors and atomic bombs—with very different containment strategies.
9. The Impact Radius: Understanding the Destruction
The effects of a nuclear explosion like Little Boy’s are catastrophic. Here’s how the blast zones are typically described:
| Zone | Approximate Size | Impact |
|---|---|---|
| Fireball (Ground Zero) | 0.36 km² | Total vaporization, incineration, complete destruction |
| Severe Blast Damage | 4.5 km² | High fatalities, buildings leveled |
| Moderate Damage | 8.7 km² | Structural damage, intense radiation, injury |
| Light Damage | 11 km²+ | Broken windows, fires, psychological trauma |
10. The Science Behind the Destruction
While conventional explosives rely on chemical reactions, nuclear weapons unleash the binding energy of atoms. That’s why even a small amount of uranium or plutonium can produce a blast equivalent to thousands of tons of TNT.

Conclusion: From Ore to Oppenheimer
Uranium’s journey from deep inside the Earth to the center of a nuclear explosion is one of science’s most sobering transformations. What starts as a rock becomes a gas, then a refined isotope, and eventually—depending on intent—a fuel for power or a tool of destruction.
Thanks to the dedication of physicists, engineers, and animators alike, we can now better visualize the chain of events and mechanisms that define this process. Whether for education, reflection, or policy, understanding these systems is crucial in our increasingly nuclear-aware world.